ERP Solutions for
Pharmaceutical, Chemical, Cosmetic Industries
Compliance with Regulations and Certifications, Guaranteed Traceability, and Established BPMN
Fill out the form to request information

The ERP Solution for Pharmaceutical, Chemical, and Cosmetic Industries
Fluentis ERP Pharma is purpose-built for the Pharmaceutical, Chemical, and Cosmetic industries, including contract manufacturers, active ingredient producers, and nutraceutical companies, where regulatory compliance is a critical operational requirement.
With FDA certification, Fluentis ERP Pharma ensures compliance with EU GMP Annex 11 and aligns with key regulatory frameworks like CFR 21 Part 210 and CFR 21 Part 211. The platform supports IT system validation processes and adheres to rigorous Good Manufacturing Practice (GMP) standards.
Fluentis ERP Pharma is designed to streamline both back-office and operational processes. It offers full lifecycle batch management, efficient procurement and production workflows, audit trail tracking, role-based access configuration, and comprehensive Batch Record management. These capabilities meet the industry’s stringent requirements for regulatory compliance and operational accuracy.
Notable companies such as Ibsa, Vevy Europe, Stallergenes, and Alpex Pharma have trusted Fluentis ERP to enhance their operations with its robust, compliance-driven solutions.

Enhanced productivity and compliance with all current regulatory standards
The pharmaceutical and chemical sectors are highly complex and regulated industries. To remain competitive in a constantly changing market, comply with strict regulatory requirements, and face global competition, pharmaceutical companies need advanced technology solutions that support every aspect of their operations and production processes.
An ERP system tailored for the pharma industry is essential for managing key areas such as research and development, planning and scheduling, production, quality control, inventory management, cost tracking, and ensuring full traceability—all while remaining compliant with GMP guidelines.
Fluentis ERP Pharma solutions help businesses:
Gain a comprehensive view of all processes
Improve batch record management
Ensure Quality Control standards are consistently met
Stay compliant with industry regulations
Track inventory levels to ensure timely order fulfillment and delivery
Increase flexibility and productivity in production processes
Streamline coordination between the Supply Chain and Distribution
Are you in this industry and facing a specific challenge that's impacting your efficiency?
See what we can do for you
Send us your RequestStrengths of Fluentis ERP Software for the Pharmaceutical, Chemical, and Cosmetic Industries:
Batch Management
Batch Record Management
QC and QA Management
Audit Trail
Authorization for Bill of Materials (BOM), Production Cycle, Items, Batch Records, and Various Documents (Purchase Orders, Payments, etc.)
Packaging and Labeling
Lot Control with Supervisor
Substance Strength Management
Production Campaign Management
Edition Management
Supplier Item Relationship Management
Password Management
Batch Traceability 360°
Production Workflow
Supervisor
Complete Integration of Administrative Processes
Integration with Measuring Instruments (LIMS)
Integrated Solution for Corporate Groups
Integrated Cost Management Control
Integrated Logistics and WMS
Resource Planning
Batch Management
Batch management is one of the most effective ways to organize operational processes. Raw materials, work-in-progress, and shipping units are identified by creating unique batches, which can be distinguished by creation date, origin, or other criteria. Fluentis integrates batch management with UDC (Unit of Cargo) management, providing companies with the ability to maintain traceability between finished products and raw materials.
With these management systems in place, it’s much easier to ensure data accuracy and track material flow throughout operational processes. ERP efficiency is further enhanced from a logistics standpoint when combined with an integrated WMS (Warehouse Management System) like Fluentis Mobile.
Proper batch identification and organizing operational processes are critical for industries where traceability is a key requirement. Fluentis focuses on balancing precision with functionality to ensure both traceability and operational speed. To achieve this, we have developed BPMN (Business Process Model and Notation) processes to offer the best use cases for our software and its capabilities.
Batch Record Management
In Fluentis, Batch Record management involves all activities related to the creation, completion, review, approval, and archiving of documents that record all operations and data related to the production of a batch of pharmaceuticals. The Batch Record is a mandatory document for compliance with Good Manufacturing Practice (GMP) regulations and ensures product traceability.
Managing the Batch Record requires collaboration between various professionals, including the production technician, quality manager, pharmacist, and medical officer. The Batch Record can be handled either in paper or electronic form. Fluentis integrates the quality management module with the production module, optimizing the preparation of the Batch Record among the various involved parties.
QC and QA Management
Fluentis manages Quality Assurance (QA) and Quality Control (QC) rights to ensure the safe release of batches at various stages of the procurement and production process. With role-based access controls for each function, Fluentis manages document approval and signing permissions, such as article inspection, batch records, certificates of conformity, and batch release approvals. This approach helps guarantee that only authorized personnel can approve critical documents, ensuring compliance and maintaining the integrity of the product quality throughout the manufacturing process.
Audit Trail
An audit trail in these industries is a secure electronic record, generated by Fluentis, with date and time stamps, that allows for the reconstruction of events related to the creation, modification, or deletion of electronic data. The audit trail is a crucial requirement in these sectors to ensure transparency, data integrity, and security of information related to the quality of pharmaceutical products, while also demonstrating compliance with applicable regulations.
The audit trail is part of the GMP documentation and is a regulatory requirement under FDA CFR 21 Part 11 and EU Annex 11. An audit trail in Fluentis records the following information: who performed the action, what was done, when it was done, and why it was done. It must be readable, accessible, and verifiable. Fluentis allows for the easy configuration of the audit trail in just a few steps, enabling users to select the specific properties they wish to monitor.
Authorization for Bill of Materials (BOM), Production Cycle, Items, Batch Records, and Various Documents (Purchase Orders, Payments, etc.)
In Fluentis, authorization is managed through a workflow that can include multiple status steps and various roles. The process may or may not require a password, and at the moment of authorization, a version of the document can also be archived. Below are the key documents typically subject to such authorizations:
Bill of Materials (BOM)
The Bill of Materials (BOM) is a comprehensive list of all the materials and components (such as packaging and raw materials) needed to produce a pharmaceutical product. The process of BOM authorization is critical for ensuring the safety, compliance, and accuracy of the final product. This process typically involves cross-functional collaboration between departments like production and quality control to verify that all necessary components are correctly listed and meet regulatory standards.
Authorizing a BOM also includes reviewing and approving production procedures and associated quality control checks, ensuring that all processes align with industry regulations and guidelines. This rigorous approval process helps guarantee that the product is manufactured to the highest standards and fully compliant with pharmaceutical regulations.
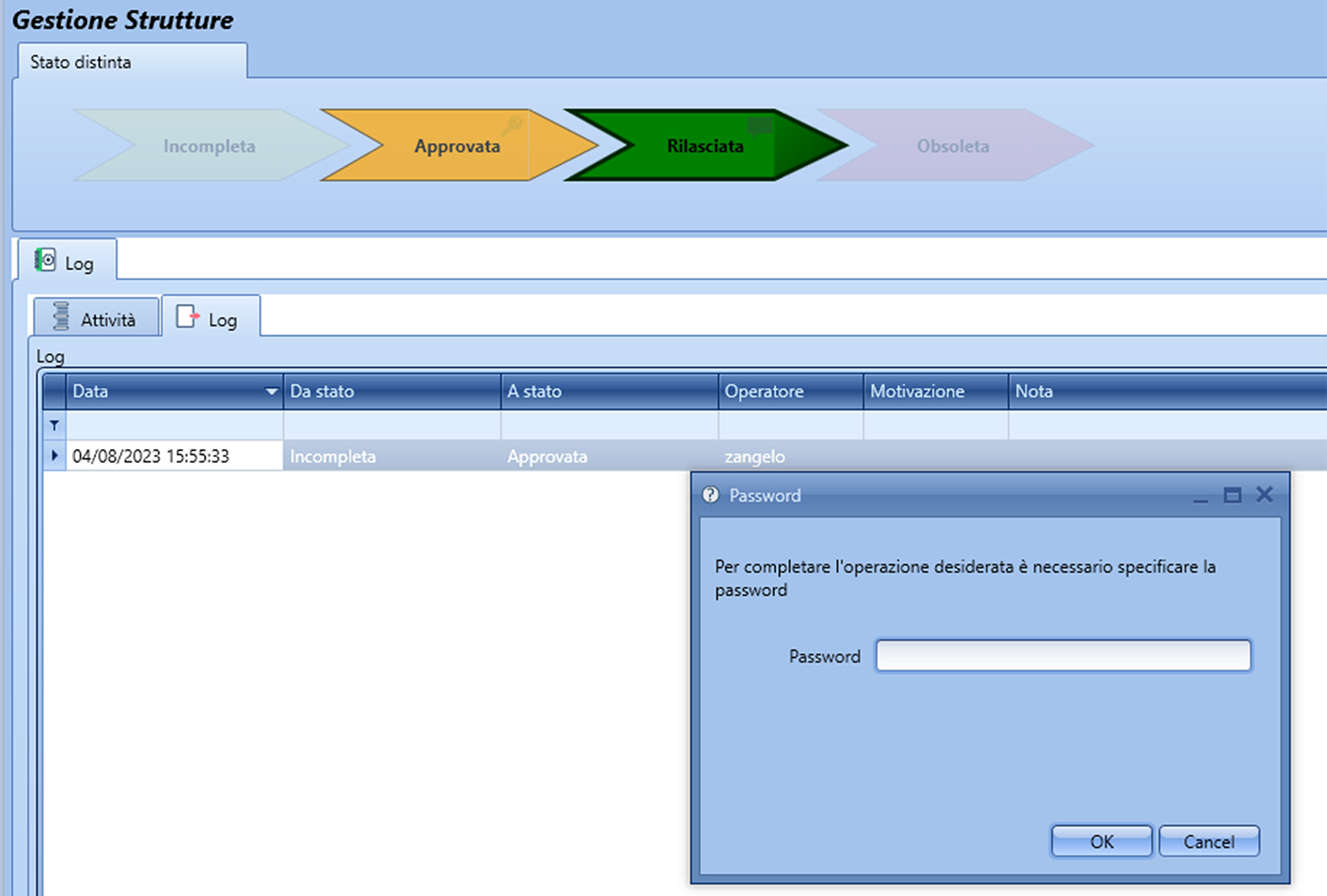
Production Cycles
In the pharmaceutical sector, production cycles define the detailed instructions for manufacturing a specific pharmaceutical product. This authorization process is crucial to ensure that production adheres to established guidelines and regulatory standards.
Production cycles must be approved by various departments, including production, quality control, and other key personnel involved in the process. The review of production instructions and process parameters—such as temperature, pressure, and mixing times—is a vital part of the authorization process to ensure product consistency and compliance with regulatory requirements. This ensures that the final product meets both safety and quality standards, adhering to industry regulations throughout the production cycle.
Items
In the pharmaceutical context, “items” include raw materials, components, and finished products. The authorization process for items focuses on the qualification of raw material suppliers, safety data assessments, and ensuring that finished products meet established specifications.
Item authorization involves several departments, such as purchasing, quality control, and regulatory affairs, to ensure that the items used in production meet high standards and comply with industry regulations. This process is essential for guaranteeing that all materials, from raw ingredients to final products, are safe, effective, and compliant with both internal and external standards before being used in production.
Batch Record
As previously mentioned, the management of the Batch Record in Fluentis involves the creation, completion, review, approval, and archiving of documents that capture all operations and data related to the production of a pharmaceutical batch. The Batch Record is a critical, compliance-required document under Good Manufacturing Practices (GMP) and is essential for ensuring product traceability.
Purchase Orders
The approval process for purchase orders—whether for all or some orders above a certain value—can be required from a responsible manager before the confirmation is sent to the supplier. Fluentis can also check that the relationship between each item and the specific supplier has been authorized.
In summary, authorization processes in the pharmaceutical sector for Bills of Materials (BOM), production cycles, and items are critical to ensuring the quality, safety, and compliance of pharmaceutical products. These processes involve multiple departments and require careful review, approval, and documentation to ensure consistency and reliability of the finished product.
Packaging and Labeling
In the pharmaceutical industry, the processes involved in transferring bulk production, packaging, and labeling are critical for ensuring proper preparation, packaging, and accurate labeling of pharmaceutical products. These procedures are essential to guarantee the quality, safety, and compliance of drugs before their distribution to the market.
The three phases are managed with physical data capture in the production area through simplified steps, allowing operators to input only essential data. This results in labels that ensure product traceability and accurate identification, a fundamental aspect for pharmaceutical products.
The processes for bulk production transfer, packaging, and labeling in the pharmaceutical sector require strict quality controls, accurate documentation, and reviews by the production, quality, and regulatory departments. All of these steps are recorded in the Batch Record.
Lot Control with Supervisor
With Fluentis’ Supervisor tool, it’s possible to manage expiration alerts for batches, which are vital in the pharmaceutical industry to ensure that pharmaceutical products and associated materials are used before their expiration dates. This control is achieved by recording the expiration date of the batch, which can be calculated in Fluentis based on the validity period set in the item’s batch master data. Alerts can be managed through email or Fluentis tasks, set at specific intervals before the expiration date, ensuring that operators are notified of the impending expiration.
Substance Strength Management
The strength of a substance refers to the weight percentage of the active ingredient in a pharmaceutical preparation. This strength is determined through specific analytical methods that must be validated and comply with the standards of the Italian Official Pharmacopoeia or other recognized pharmacopoeias. The strength of a substance is crucial for ensuring the quality, efficacy, and safety of medicines, as well as for calculating the correct dosage to administer to patients.
A substance’s strength can vary over time due to factors like aging, storage conditions, interactions with other components, or with the container. For this reason, medicines should be stored according to the instructions on the package insert and used before the expiration date.
In Fluentis, managing the strength of components within a given formulation allows for the automatic recalculation of the amount of excipient to be used.
Production Campaign Management
Production campaign management is a process that involves planning, executing, monitoring, and optimizing a company’s manufacturing activities. It entails coordinating and optimizing the human, material, and energy resources needed to efficiently and effectively produce goods or services.
In Fluentis, a production campaign is typically managed for a specific product and is divided into multiple production orders just before the campaign’s start date. This ensures that resources are aligned and the production flow is streamlined from the beginning.
Edition Management
One of the key records managed in the pharmaceutical sector is the package leaflet (or product insert) for a medication. This document contains essential information to ensure the safe and proper use of the product, including its composition, indications, dosage, contraindications, side effects, and storage instructions.
The package leaflet is approved by the Italian Medicines Agency (AIFA) or the European Commission and is an integral part of the Marketing Authorization (MA) for the product. It may be updated throughout the product’s lifecycle based on new scientific evidence or regulatory requirements.
In Fluentis, the system handles the “Edition” master data for items like package leaflets, cartons, labels, etc. Each edition is linked to a specific product batch. Once the edition is assigned to a batch and stored in inventory, it will be included in the necessary printouts, and if no longer valid, the corresponding batch will not be available for picking.
Supplier Item Relationship Management
In the pharmaceutical industry, validating an item for a specific supplier means ensuring that the purchased product meets the required quality, safety, and efficacy standards outlined by both the customer and regulatory authorities. Validation can apply to active pharmaceutical ingredients (APIs), finished pharmaceutical forms, or packaging materials. The validation process relies on appropriate analytical methods, which must be developed and validated according to scientific and regulatory standards.
These analytical methods assess the chemical, physical, biological, and microbiological properties of the item, as well as any potential impurities or contaminants.
Additionally, the validation process includes verifying that the supplier complies with Good Manufacturing Practices (GMP) and any other certifications required by the customer or regulatory agencies.
The validation process involves multiple professionals, such as laboratory technicians, quality managers, pharmacists, and medical experts. It is essential to ensure the quality of the final product and safeguard patient health.
In Fluentis, the validation process is managed through the Quality and Purchasing modules, with a workflow that tracks every specific supplier-item relationship.
Password Management
In the pharmaceutical sector, password management plays a critical role in ensuring the security and integrity of sensitive health data and product quality information. Passwords are one of the primary tools used for user authentication and authorization to access systems and devices that handle such data.
Password management must also comply with relevant regulations on data protection, such as the General Data Protection Regulation (GDPR), and the requirements from regulatory authorities like FDA CFR 21 Part 11 and EU Annex 11. These regulations demand that passwords are adequately protected from unauthorized access, that user activities are tracked through Audit Trails, and that the responsibilities of both users and IT system providers are clearly defined.
In Fluentis, password requests can be set for any function, adding an extra layer of security alongside the user role permissions defined for each specific function. For instance, when changing a batch status, the system will typically require a password to ensure the procedure is secure.
Batch Traceability 360°
Batch traceability is a critical requirement in the pharmaceutical industry to ensure product safety, compliance, and quality throughout the entire supply chain, from production to the end consumer.
Fluentis’ industry-specific features streamline batch traceability by allowing users to view all relevant information about a selected batch, including the materials and components used, across various documents (such as production orders, purchase orders, sales delivery notes, warehouse movements, etc.). These details are directly linked to the Bill of Materials (BOM).
Production Workflow
In Fluentis, the production workflow is managed through a dedicated Workflow system that tracks the progress of the production order status. Within this system, key processes like Batch-to-Material Assignment are triggered, enabling the reservation of batches needed to meet material demands while adhering to inventory principles such as FEFO (First Expired, First Out), FIFO (First In, First Out), and LIFO (Last In, First Out).
In this sector, managing approval workflows is critical to ensuring quality, safety, and compliance in production operations. Fluentis’ approval workflow offers several advantages:
- Ensures compliance with the strict regulations of the pharmaceutical industry.
- Controls quality and validates production processes.
- Provides traceability and accountability for every decision.
- Reduces human error and improves operational efficiency.
The workflow includes a clear hierarchy of authorizations, accurate documentation (enabled by integrated document management), and automation through specialized software (such as Fluentis Supervisor). These features collectively improve the safety, reliability, and efficiency of production processes.
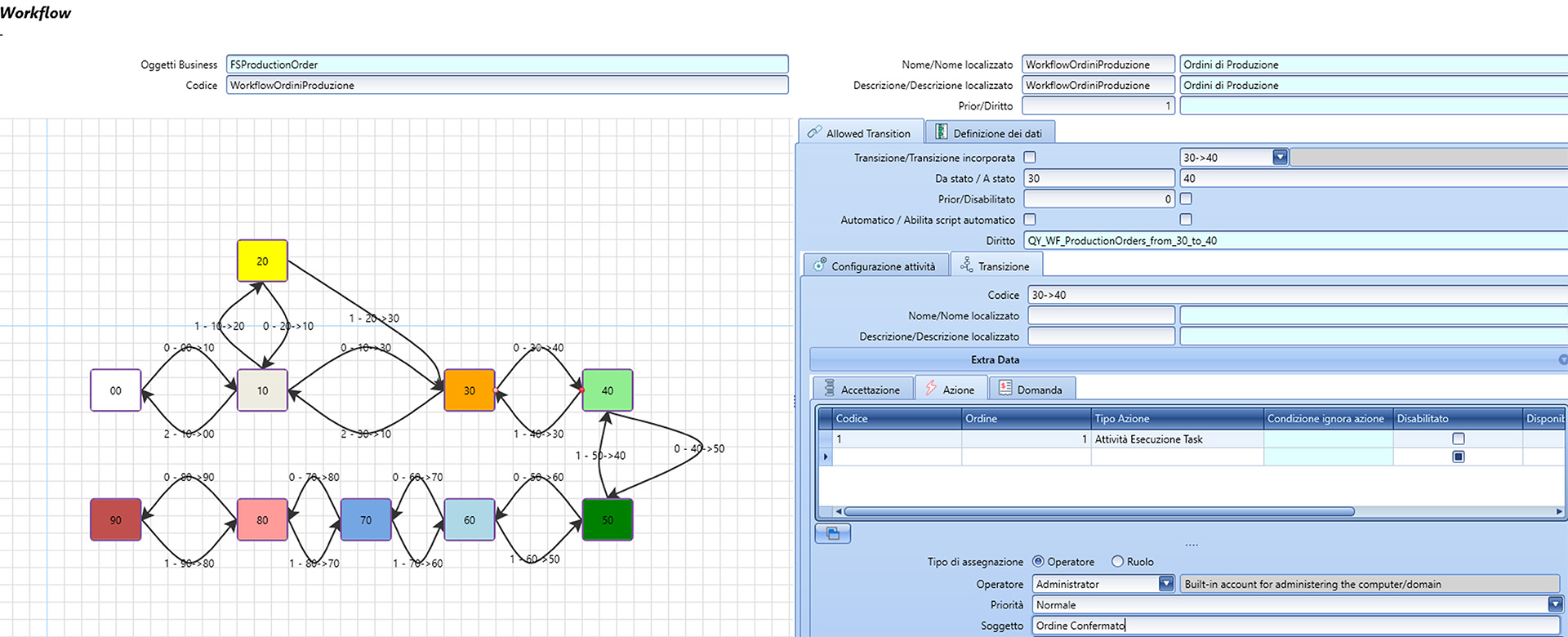
Supervisor: automatic process scheduler
Supervisor is designed to automate routine control and verification tasks that operators perform daily across various operational areas, thereby enhancing efficiency.
With its suite of algorithms, Supervisor manages a wide range of processes, including:
- Monitoring production progress,
- Overseeing inventory levels,
- Managing supplier delivery schedules,
- Tracking credit situations in administration.
By acting as an activity scheduler, Supervisor notifies users of exceptions only when they occur, utilizing email alerts for real-time updates.
Supervisor’s functionality is easily expandable via customizable VBScript-based control rules, enabling quick and simple adaptation to specific operational needs. Additionally, it communicates with operators not only via email but also through SMS notifications, using a properly configured GSM modem.
Complete Integration of Administrative Processes
The Administrative Area in Fluentis is designed to manage compliance with tax regulations, including those in Italy and some foreign tax jurisdictions. For companies operating across multiple countries, Fluentis provides a list of pre-configured international tax solutions or integrates seamlessly with specialized global tax tools.
Weighing Room Management and Integration with Measuring Instruments (LIMS)
Preparation and dispensing are fully integrated with weighing stations, ensuring vertical traceability of the weight data collected.
Integrated Solution for Corporate Groups
Fluentis solutions are natively designed for multi-company environments, even within the same database. They support multi-tax jurisdictions, multiple languages, and multi-currency operations. Additionally, Intercompany features are available to automate the creation and flow of information between companies within the corporate group.
Integrated Cost Management Control
The Cost Control module enables detailed allocation of revenues and expenses by purpose. This achieves two key goals:
- Project/Job Cost Accounting: Offers specific reporting and graphical analysis to highlight margins and profitability.
- Cost Center Accounting: Tracks costs assigned to individual departments or resources, enabling calculation of operational rates.
Additionally, this module supports interim financial statements with multiple reclassification models. It automates the generation of summarized data critical for monitoring and steering company performance, accompanied by diagrams and graphs for easier interpretation and decision-making
Integrated Logistics and WMS
Fluentis ERP PHARMA includes specialized features designed to enhance both internal and external logistics efficiency through its Fluentis Mobile WMS.
The Fluentis WMS operates in Wi-Fi-enabled environments with touchscreen devices connected to the ERP via Web Services. This integration offers real-time information, increased flexibility, speed, and reduced errors.
The Fluentis Mobile WMS defines the physical warehouse structure, organizing it into aisles, racks, shelves, or simpler layouts such as areas, yards, or production line stations, ensuring unique location identification.
Key procedures supported by the system include:
- Automating material loading and unloading processes.
- Optimizing material picking and storage logic.
- Accelerating order fulfillment and material receipt.
- Streamlining activities such as packing list creation and finished product shipping.
Resource Planning: Materials, Work Centers, and Human Resources
The planning area is equipped with algorithms to support both the commercial and planning functions.
- MRP I & II: These are used to optimize material procurement and improve resource efficiency. MRP I focuses on material requirements planning based on forecasts, while MRP II extends to include the integration of production capacity and human resources, ensuring optimal alignment of material flow with manufacturing capacity.
- DDMRP (Demand Driven Material Requirements Planning): This approach is focused on supply chain planning and execution driven by actual customer demand, offering several benefits:
- Reduction in inventory levels.
- Improved service levels.
- Decreased planning complexity.
- Enhanced competitiveness in the market.
- CRP (Capacity Resource Planning): CRP helps manage the workloads of work centers, visualized through specific Gantt charts. This ensures efficient allocation of resources and optimized production schedules.
Discover how Fluentis ERP
can transform your business
15-day free trial | No automatic renewal | Instant access
Contact us for more information
Get in touch with us if you:
- Are a SME in Manufacturing, Distribution, or Services
- Need to streamline and digitalize your business processes
- Want to take advantage of the benefits of a native cloud solution
- Want to replace your non-integrated softwares with a unified ERP platform
+1 281 404 1726
Chat with us